▎药明康德/报道

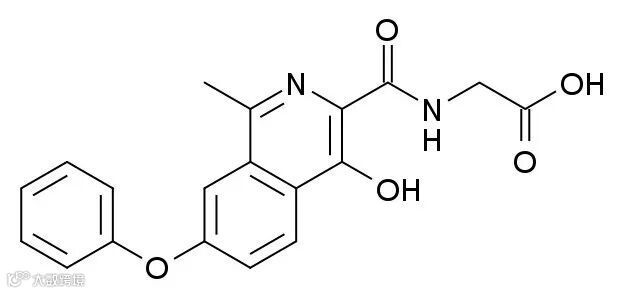
▲罗沙司他分子结构式(图片来源:Meodipt [Public domain])
参考资料:
[1] AstraZeneca agrees to buy US FDA Priority Review Voucher from Sobi. Retrieved Aug. 22, 2019, from https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2019/astrazeneca-agrees-to-buy-us-fda-priority-review-voucher-from-sobi-22082019.html
[2] Astra could use a voucher to cement roxadustat’s head start. Retrieved Aug. 22, 2019, from https://www.evaluate.com/vantage/articles/news/astra-could-use-voucher-cement-roxadustats-head-start
[3] China becomes the first country to approve roxadustat for all chronic kidney disease patients with anaemia. Retrieved Aug. 22, 2019, from https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2019/roxadustat-approved-in-china-for-the-treatment-of-anaemia-in-non-dialysis-dependent-patients-with-chronic-kidney-disease-22082019.html
[4] 中国首发!First in class新药罗沙司他正式在国内获批. Retrieved Aug. 22, 2019, from https://mp.weixin.qq.com/s/Tn6mqy9AEUlF_7ylmSp7ew
[5] 速递| 治疗肾性贫血,葛兰素史克首次在日本递交新药申请. Retrieved Aug. 22, 2019, from https://mp.weixin.qq.com/s/c9eqw_mvEJyq64TTUQbJlw
本文来自药明康德微信团队,欢迎转发到朋友圈,谢绝转载到其他平台;如有开设白名单需求,请在文章底部留言;如有其他合作需求,请联系wuxi_media@wuxiapptec.com



