
编者按:寡核苷酸药物是全球新药开发的重要热点,近年来在罕见病等多个领域得到快速应用。当前,全球共有超过300项寡核苷酸疗法相关临床试验正在进行之中,未来有望造福更多病患。为帮助合作伙伴更高效地推动寡核苷酸药物从实验室走向临床,药明康德旗下WuXi TIDES平台围绕寡核苷酸、多肽及其相关化学偶联药物建立了一体化解决方案,覆盖定制合成、共价连接、工艺开发和CMC等关键环节,赋能创新项目加速进入临床阶段。本文将盘点2025年寡核苷酸产业的重要进展,并分享一则具体赋能案例,助您了解该领域的最新动态与发展方向。
临床与监管进展
今年3月,赛诺菲(Sanofi)与Alnylam Pharmaceuticals联合开发的潜在重磅siRNA疗法Qfitlia(fitusiran)获美国FDA批准,用于治疗血友病患者。两项3期临床试验结果显示,fitusiran可将患者的年化出血率降低约90%,部分患者的给药频率还可减少至每两个月一次。几乎同期,Alnylam的另一款siRNA药物Amvuttra(vutrisiran)也获得FDA批准,用于治疗伴心肌病的转甲状腺素蛋白淀粉样变性(ATTR-CM)成人患者。
进入7月,美国FDA批准诺华(Novartis)每年两次给药的siRNA疗法Leqvio(inclisiran)扩展适应症,允许其作为单药并联合饮食控制和运动,用于降低成人高胆固醇血症患者的低密度脂蛋白胆固醇(LDL-C)水平。值得注意的是,此次标签更新源于该PCSK9靶向疗法在降低LDL-C方面的积极临床数据,是FDA主动要求进行的调整。随后在8月,FDA又批准反义寡核苷酸配体偶联(LICA)药物Dawnzera(donidalorsen),用于预防12岁及以上成人及儿童患者的遗传性血管性水肿(HAE)发作。根据新闻稿,Dawnzera是首款获批用于HAE的RNA靶向药物。今年11月,FDA批准Arrowhead Pharmaceuticals旗下靶向APOC3的“first-in-class”RNAi疗法Redemplo(plozasiran),作为饮食控制的辅助治疗,用于降低家族性乳糜微粒血症综合征成人患者的甘油三酯(TG)水平。
除药物获批,多项寡核苷酸疗法的后期临床试验数据也在今年公布。例如,安进(Amgen)开发的siRNA疗法olpasiran在一项2期试验中,可使动脉粥样硬化性心血管疾病合并高脂蛋白a——Lp(a)水平升高患者的Lp(a)浓度降低约92%,且疗效可持续超过一年,该疗法目前已进入3期临床阶段。此外,开发用以治疗代谢功能障碍相关脂肪性肝炎(MASH)的ASO疗法ION224也在今年获得积极的2期临床试验结果。有近60%的最高剂量组患者达到主要终点,显示出在MASH疾病活动上的改善,而此数值在安慰剂组仅为19%。
在罕见疾病方面,补体C5靶向siRNA疗法cemdisiran,在用以治疗成人全身性重症肌无力(gMG)的NIMBLE临床3期试验中达到试验终点。分析显示,每三个月皮下注射一次的cemdisiran单药显示出平均74%的补体活性抑制,而cemdisiran与C5抗体pozelimab的联合疗法则达成近99%的补体活性抑制。与此同时,ASO疗法zilganersen也在关键试验中显著改善罕见神经系统疾病亚历山大病(AxD)患者的步行能力。根据新闻稿,zilganersen是在AxD患者中显示出改变疾病进程影响的首款在研疗法。以上两款疗法皆预计于2026年第一季度提交相关监管申请。

研发合作与融资进展
2025年,多家大型医药企业在寡核苷酸领域持续加码,通过战略合作与并购进一步完善布局。5月,渤健(Biogen)与City Therapeutics达成一项潜在总额高达10亿美元的合作协议,初期聚焦于开发针对中枢神经系统关键靶点的新型RNAi疗法。此前在2月,渤健还与Stoke Therapeutics签署协议,就其反义寡核苷酸疗法zorevunersen的开发与商业化达成最高3.85亿美元的合作。诺华(Novartis)于4月宣布拟以最高约17亿美元收购Regulus Therapeutics。Regulus专注于靶向microRNA的疗法开发,其核心产品farabursen是一款靶向miR-17的下一代寡核苷酸药物,用于治疗常染色体显性多囊肾病(ADPKD),并已完成一项1b期多剂量递增临床试验。
今年9月,诺华接连达成两项大额siRNA疗法授权合作。其一,诺华与Arrowhead Pharmaceuticals就后者开发的α-突触核蛋白靶向siRNA疗法ARO-SNCA签署总额高达20亿美元的全球许可与合作协议。该疗法目前处于临床前阶段,拟用于治疗包括帕金森病在内的突触核蛋白病。其二,诺华与Argo Biopharma围绕多项心血管siRNA管线达成合作,交易总额最高可达52亿美元,进一步夯实其在心血管代谢领域的布局。
展望未来,寡核苷酸疗法有望成为一类改变医学格局的关键治疗模式。作为该领域的先驱之一,Alnylam公司创始首席执行官John Maraganore博士在接受美国化学会旗下C&EN杂志采访时表示,未来医学发展亟需包括寡核苷酸在内的更复杂治疗手段,以更好地满足不同患者的临床需求。这类新型分子疗法有望在多个疾病领域发挥关键作用。我们期待这些前沿技术不断取得突破,早日转化为真正惠及全球患者的创新药物。
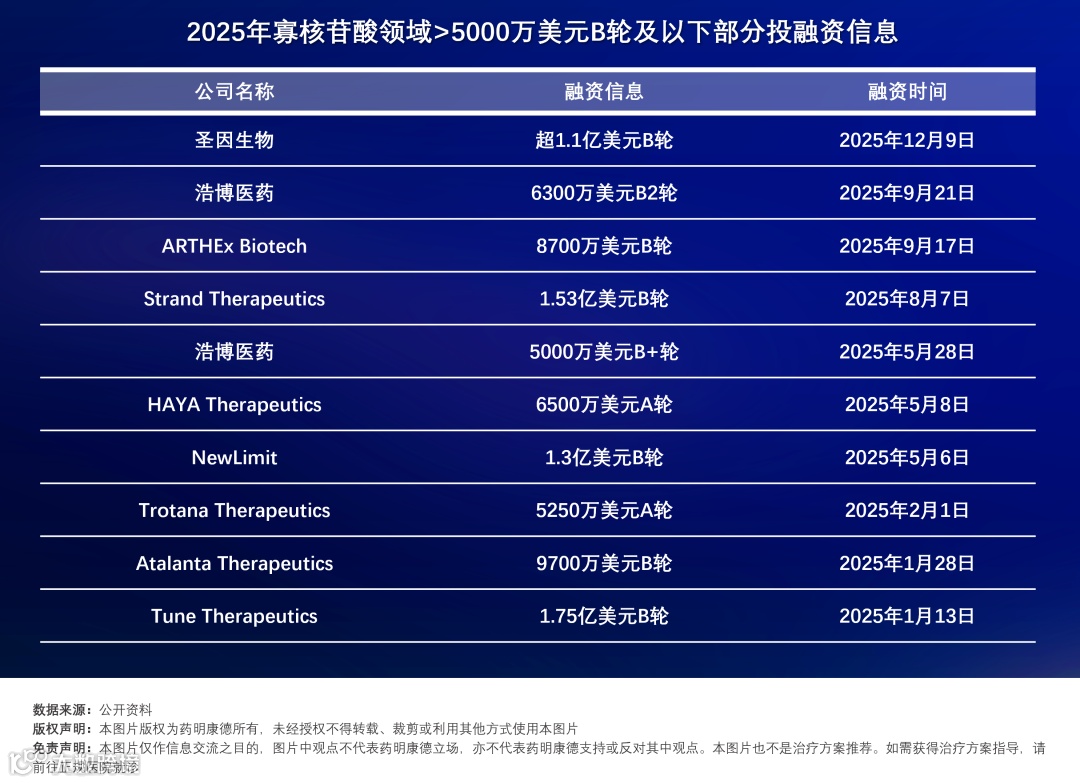
▲2025年寡核苷酸领域>5000万美元B轮及以下部分投融资信息
一体化平台高效赋能寡核苷酸药物研发
作为医药创新的赋能者,药明康德化学业务旗下WuXi TIDES平台围绕siRNA、ASO等寡核苷酸疗法,建立了化合物合成、工艺开发及生产的一站式服务平台,覆盖从药物发现、CMC开发,到商业化生产的全生命周期,加速将合作伙伴的创新构想转化为现实,更好地造福全球病患。以下案例将展示WuXi TIDES的一体化平台如何加速合作伙伴ASO药物的开发进程。
2023年,一家生物技术公司与WuXi TIDES合作进行ASO药物的早期筛选研究,WuXi TIDES的药物化学团队为其提供了超过400种携带骨架化学修饰的ASO化合物,以协助确定最具前景的分子。然而,早期研究发现,创新骨架修饰导致候选化合物中出现新的杂质。在最初的合成过程中,这些杂质占比高达25%,不仅降低了产率和纯化效率,还可能带来潜在毒性,给后续临床开发带来挑战。
面对这一难题,WuXi TIDES药物化学团队和工艺研发团队密切配合,从两个方向入手解决问题。一方面,药物化学团队与合作伙伴共同探索杂质产生的潜在原因,设计出定制化的amidite和分子砌块,规避杂质产生的关键合成机制,并快速生产这些新分子砌块,协助工艺研发团队加速验证工艺设计策略,以有效地控制杂质。此外,工艺研发团队通过优化工艺参数,系统性地降低了杂质的产生。最终,经过持续工艺优化,杂质占比成功从25%降低至5%,同时最终收率也从最初的0.5 g/mol提高到3.4 g/mol。
在该项目中,WuXi TIDES各团队高效协作,不仅在12个月内完成了先导化合物的优化、工艺开发及GMP生产,更帮助合作伙伴基于数据进行快速决策,选出综合效力、稳定性和开发潜力俱佳的ASO候选化合物,为后续临床研究奠定了坚实基础。随着越来越多的ASO以及其他寡核苷酸药物进入临床开发,这种产业协同模式将成为加快研发步伐的重要推动力。
Multiple FDA Approvals for Oligonucleotide Therapeutics in 2025
Oligonucleotide-based therapeutics continue to stand out as a key area in global drug development, with rapid progress seen across rare diseases and beyond. Currently, over 300 oligonucleotide clinical trials are ongoing worldwide, offering hope to a growing number of patients. To support partners in efficiently advancing these innovative therapies from discovery to clinic, WuXi TIDES offers efficient, flexible, and high-quality solutions for the drug development of oligonucleotides, peptides and related synthetic conjugates (“TIDES” drugs). The platform greatly simplifies the TIDES drug development by providing all discovery, CMC development and the entire manufacturing supply chain under one roof. Here we summarize key developments in the oligonucleotide space in 2025 and share a case study that illustrates how WuXi TIDES helps accelerate progress in this dynamic area.
Clinical and Regulatory Progress
In March this year, Qfitlia (fitusiran), a potentially best-in-class siRNA therapy jointly developed by Sanofi and Alnylam Pharmaceuticals, received approval from the U.S. Food and Drug Administration (FDA) for the treatment of patients with hemophilia. Results from two Phase 3 clinical trials demonstrated that fitusiran reduced annualized bleeding rates by approximately 90%, with dosing frequency reduced to once every two months in a subset of patients. Around the same time, Amvuttra (vutrisiran), another siRNA therapy from Alnylam, was also approved by the FDA, becoming the first RNAi therapeutic indicated for the treatment of adult patients with transthyretin amyloid cardiomyopathy (ATTR-CM).
In July, the FDA approved an expanded indication for Leqvio (inclisiran), a twice-yearly siRNA therapy from Novartis, allowing its use as monotherapy in combination with diet and exercise to reduce low-density lipoprotein cholesterol (LDL-C) levels in adults with hypercholesterolemia. Notably, this label update was initiated by the FDA based on positive clinical data demonstrating LDL-C reduction with this PCSK9-targeting therapy. Subsequently, in August, the FDA approved Dawnzera (donidalorsen), an antisense oligonucleotide LIgand-Conjugated Antisense (LICA) therapy, for the prevention of hereditary angioedema (HAE) attacks in adults and pediatric patients aged 12 years and older. According to the company, Dawnzera is the first RNA-targeted therapy approved for HAE. In November, the FDA approved Redemplo (plozasiran), a first-in-class APOC3-targeting RNAi therapy developed by Arrowhead Pharmaceuticals, as an adjunct to diet to reduce triglyceride (TG) levels in adults with familial chylomicronemia syndrome.
Beyond regulatory approvals, several late-stage clinical readouts for oligonucleotide-based therapies were also reported this year. In a Phase 2 study, olpasiran, an siRNA therapy developed by Amgen, reduced lipoprotein(a) [Lp(a)] levels by approximately 92% in patients with atherosclerotic cardiovascular disease and elevated Lp(a), with effects sustained for more than one year. The program has since advanced into Phase 3 development. In parallel, ION224, an antisense oligonucleotide therapy under development for metabolic dysfunction-associated steatohepatitis (MASH), reported positive Phase 2 results. Nearly 60% of patients in the highest-dose group achieved the primary endpoint, demonstrating improvement in MASH disease activity, compared with 19% in the placebo group.
In the rare disease space, the complement C5-targeting siRNA therapy cemdisiran met its primary endpoint in the Phase 3 NIMBLE trial evaluating treatment in adults with generalized myasthenia gravis (gMG). Data showed that cemdisiran monotherapy, administered via subcutaneous injection once every three months, achieved an average 74% inhibition of complement activity, while combination therapy with the C5 antibody pozelimab resulted in nearly 99% complement inhibition. Meanwhile, the antisense oligonucleotide therapy zilganersen demonstrated significant improvement in walking ability in patients with the rare neurological disorder Alexander disease (AxD) in a pivotal trial. According to company disclosures, zilganersen is the first investigational therapy to show potential disease-modifying effects in AxD. Both programs are expected to submit regulatory applications in the first quarter of 2026.
R&D Collaboration & Financing Progress
In 2025, major pharmaceutical companies continued to deepen their commitment to the oligonucleotide space through strategic partnerships and acquisitions. In May, Biogen entered into a collaboration with City Therapeutics with a potential total value of up to $1 billion, initially focused on developing novel RNAi therapies targeting key central nervous system targets. Earlier in February, Biogen also signed a collaboration agreement with Stoke Therapeutics, valued at up to $385 million, for the development and commercialization of the antisense oligonucleotide therapy zorevunersen.
In April, Novartis announced its intention to acquire Regulus Therapeutics for up to approximately $1.7 billion. Regulus specializes in microRNA-targeted therapies, and its lead asset farabursen, a next-generation oligonucleotide targeting miR-17, is being developed for the treatment of autosomal dominant polycystic kidney disease (ADPKD). The program has completed a Phase 1b multiple-ascending-dose clinical trial.
In September, Novartis further expanded its siRNA portfolio through two major licensing transactions. First, Novartis entered into a global licensing and collaboration agreement with Arrowhead Pharmaceuticals for ARO-SNCA, an α-synuclein-targeting siRNA therapy, with a total deal value of up to $2 billion. The therapy is currently in preclinical development and is intended for the treatment of synucleinopathies, including Parkinson’s disease. Second, Novartis formed a broad collaboration with Argo Biopharma covering multiple cardiovascular siRNA programs, with a potential total transaction value of up to $5.2 billion, further strengthening its strategic position in cardiovascular and metabolic diseases.
Looking ahead, oligonucleotide therapies are poised to emerge as a transformative class of treatments with the potential to reshape the medical landscape. As one of the pioneers in the field, Dr. John Maraganore, founding Chief Executive Officer of Alnylam, noted in an interview with C&EN, the magazine of the American Chemical Society, that the future of medicine will require more sophisticated therapeutic modalities—including oligonucleotides—to better address the diverse clinical needs of patients. These novel molecular therapies are expected to play a critical role across a wide range of disease areas. We look forward to continued breakthroughs in these cutting-edge technologies and to their rapid translation into innovative medicines that deliver meaningful benefits to patients worldwide.
WuXi TIDES Accelerates Oligonucleotide Drug Development for Global Partners
WuXi TIDES has built an end-to-end service platform for oligonucleotide therapeutics, including siRNA and ASO, encompassing compound synthesis, process development, and manufacturing. Covering the full lifecycle—from drug discovery and CMC development to commercial production—the platform enables partners to rapidly transform innovative ideas into reality and bring benefits to patients worldwide. The following case study highlights how WuXi TIDES’ fully integrated platform is accelerating the development of an ASO therapy for one of our partners.
In 2023, a biotech company partnered with WuXi TIDES to conduct early-stage ASO screening. The discovery synthesis team undertook extensive SAR (Structure-Activity Relationship) exploration—screening more than 400 ASO variants with various types of backbone and ribose modifications to help identify the most promising candidates. However, early-stage studies revealed that novel backbone modifications introduced new impurities—up to 25% in some initial batches—significantly lowering yield and purification efficiency, while raising concerns about potential toxicity that could hinder clinical development.
To address these challenges, WuXi TIDES’ Discovery Chemistry team and Process Development (PRD) team collaborated closely on two fronts. The Discovery Chemistry Team worked with the client to investigate the source of impurities and designed specialized amidites and building blocks to circumvent the pathway leading to key impurities. In parallel, the PRD team rapidly synthesized these components and supported swift validation of the optimized strategy. Additionally, the PRD team systematically optimized multiple process parameters to further reduce impurities.
Ultimately, through a series of process refinements, the impurity level was reduced from 25% to just 5%, and the final yield increased from 0.5 g/mol to 3.4 g/mol.
Thanks to efficient cross-functional collaboration, WuXi TIDES completed hit-to-lead optimization, process development, and GMP manufacturing within 12 months. The partner was able to make data-driven decisions and select a lead ASO candidate with optimal potency, stability, and development potential—laying a strong foundation for clinical studies. As more ASO therapies enter development, this model of collaborative development will be critical for accelerating future breakthroughs.
Advances in chemical modification and delivery technology have enabled oligonucleotide therapeutics to reach previously inaccessible tissue targets—offering new hope for rare and hard-to-treat diseases. Looking ahead, the continued evolution of this field is expected to deliver more innovative treatments to benefit patients worldwide. WuXi TIDES remains committed to leveraging its integrated CRDMO platform to empower the development of oligonucleotide therapeutics, helping partners translate scientific innovation into life-changing medicines.
参考资料:
[1] Oligonucleotide Synthesis Market Industry Trends and Global Forecasts Report 2025-2035: Market Strengthens With 110+ Providers, North America Leads, Clinical Pipeline Exceeds 300 Trials - ResearchAndMarkets.com. Retrieved December 21, 2025 from https://www.businesswire.com/news/home/20251203559059/en/Oligonucleotide-Synthesis-Market-Industry-Trends-and-Global-Forecasts-Report-2025-2035-Market-Strengthens-With-110-Providers-North-America-Leads-Clinical-Pipeline-Exceeds-300-Trials---ResearchAndMarkets.com
[2] Olezarsen significantly reduces triglycerides and acute pancreatitis events in landmark pivotal studies for people with severe hypertriglyceridemia (sHTG). Retrieved September 30, 2025 from https://ir.ionis.com/news-releases/news-release-details/olezarsen-significantly-reduces-triglycerides-and-acute
[3] Biogen’s Investigational Tau-Targeting Therapy BIIB080 Receives FDA Fast Track Designation for the Treatment of Alzheimer’s Disease. Retrieved June 18, 2025 from https://investors.biogen.com/news-releases/news-release-details/biogens-investigational-tau-targeting-therapy-biib080-receives
[4] Alys Pharmaceuticals Announces Dosing of First Patient in Phase IIa Trial of ALY-101 for Alopecia Areata. Retrieved June 18, 2025 from https://alyspharma.com/alys-pharmaceuticals-announces-dosing-of-first-patient-in-phase-iia-trial-of-aly-101-for-alopecia-areata/
[5] Is this the decade of RNA? Retrieved June 19, 2025 from https://cen.acs.org/pharmaceuticals/decade-RNA/97/web/2019/01?utm_source=chatgpt.com
[6] Arrowhead Pharmaceuticals and Novartis Enter into a Global License and Collaboration Agreement. Retrieved October 1, 2025 from https://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-pharmaceuticals-and-novartis-enter-global-license-and
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。

分享,点赞,在看,聚焦全球生物医药健康创新


