OECD No.22号文对于混合系统的审核要求如下:
Increased data review is likely to be required for hybrid systems because they are vulnerable to non-attributable data changes.All records from hybrid systems that are defined by the data set should be reviewed by a qualified person.
混合系统很可能需要加强数据审核,因为它们容易发生无法追溯责任人的数据更改。应由有资质的人员对混合系统中由数据集定义的所有记录进行审核。
指南仅用一个词“non-attributable data changes”来描述审核混合系统的原因,作为审核人员读了这个要求对于该审核什么,怎么审核一脸茫然,仔细读读下面来自数据可靠性大师的文章吧。
Data Integrity Focus, Part III: What Is the Problem with Hybrid Systems?
数据完整性聚焦,第三部分:混合系统的问题何在?
A hybrid system is the worst possible choice for managing your regulated data.
混合系统是管理受监管数据最糟糕的选择。
Regulatory authorities globally have concerns about hybrid computerized systems. These are the worst possible computerized system to have in your laboratory from a regulatory perspective. Here, we discuss what a hybrid system is, and explain why there is such a fuss about them.
全球监管机构对混合型计算机化系统均表示关注。从监管角度来看,这类系统是实验室中最不可取的计算机化系统。本文将阐述什么是混合系统,并解释为何对此类系统存在如此多的争议。
This article is the third in a six-part series dealing with data integrity. The first discussed a data integrity model to present the scope of data integrity and data governance program for an organization (1). The second discussed data process mapping to identify data integrity gaps in a process involving a chromatography data system (CDS), and looked at ways to remediate these gaps (2). The CDS was described originally as a hybrid system, and I mentioned that this subject would be the topic of this third article.
本文是关于数据完整性的六部分系列文章中的第三篇。第一篇介绍了一个数据完整性模型,用以界定组织在数据完整性及数据治理项目中的范围(1);第二篇讨论了数据流程映射,用于识别色谱数据系统(CDS)相关流程中的数据完整性漏洞,并探讨了弥补这些漏洞的方法(2)。该CDS最初被描述为一个混合系统,我当时提到,这一主题将成为本篇第三篇文章的讨论重点。
What is a Hybrid System?
什么是混合系统?
First, it is important to define what we mean by a hybrid computerized system. The best definition and description is found in the World Health Organization (WHO) guidance (3):
首先,明确我们所说的“混合型计算机化系统”至关重要。世界卫生组织(WHO)指南(3)中提供了最清晰的定义和描述:
This refers to the use of a computerized system in which there is a combination of original electronic records and paper records that comprise the total record set that should be reviewed and retained.
这指的是使用一种计算机化系统,其中包含构成应被审查和保存的完整记录集的原始电子记录和纸质记录的组合。
An example of a hybrid approach is where laboratory analysts use computerized instrument systems that create original electronic records and then print a summary of the results.
混合方法的一个示例是,实验室分析人员使用能够生成原始电子记录的计算机化仪器系统,然后打印结果摘要。
The hybrid approach requires a secure link between all record types, including paper and electronic, throughout the records retention period.
混合方法要求在记录保存期间,所有类型的记录(包括纸质和电子记录)之间保持安全的关联。
Where hybrid approaches are used, appropriate controls for electronic documents, such as templates, forms and master documents, that may be printed, should be available.
在使用混合方法时,应具备针对可能被打印的电子文档(如模板、表单和主文档)的适当控制措施。
A schematic of a hybrid computerized system is shown in Figure 1. There are a number of electronic records within the computerized system, and these must be securely linked with the paper printouts that are signed by the analyst and reviewer. Often, the focus is only on the paper printout and not the electronic records when, in fact, it should be the other way around; e-records are the critical data, and signed paper printouts are only a small part of the records.
混合型计算机化系统的示意图如图1所示。在该计算机化系统中存在大量电子记录,这些电子记录必须与由分析人员和审核人员签署的纸质打印件安全地关联起来。通常,人们往往只关注纸质打印件而忽视了电子记录,而事实上情况恰恰相反:电子记录才是关键数据,而签署的纸质打印件仅是记录中的一小部分。(关注点1:如何从纸质记录追溯到电子记录?从图1可知打印件体现电子记录文件名)


Figure 1: A schematic of a hybrid system consisting of electronic records and signed paper printouts (4).
图1:由电子记录和已签名的纸质打印件组成的混合系统的示意图(4)。
Unable Able Laboratories
Able实验室
Able Laboratories is the classic data integrity case study that can be summarized as "You can't falsify data into compliance." This now defunct generic pharmaceutical company had passed several US Food and Drug Administration (FDA) inspections before a whistleblower called to alert the agency about fraudulent quality control (QC) work. Inspectors quickly found that data were being falsified on an industrial scale, using a variety of means such as copy and paste, manipulation of weights, and chromatographic integration (5). The heart of the falsification effort was a CDS in which the audit trail that could not be turned off identified who had falsified which data and when. The reason why the agency had missed the falsification is that the inspectors focused on paper printouts alone. As a result, there were changes to the way FDA and other regulatory agencies inspect computerized systems with updated regulations (6,7) and guidance (3, 8–11), as we will discuss now.
Able Laboratories 是一个典型的数据完整性案例研究,其教训可总结为:“你无法通过伪造数据来实现合规。” 这家现已倒闭的仿制药公司曾在多次美国食品药品监督管理局(FDA)检查中过关。然而,一名举报人向该机构举报了其欺诈性的质量控制(QC)工作后,检查人员很快发现,该公司正以工业规模伪造数据,手段多种多样,包括复制粘贴、篡改称重数值以及色谱积分处理等。造假行为的核心是一个色谱数据系统(CDS),该系统中的审计追踪功能无法被关闭,因此清楚地记录了是谁在何时伪造了哪些数据。FDA 之前未能发现这些造假行为的原因在于,检查人员当时仅关注了纸质打印件。这一事件直接导致了 FDA 及其他监管机构改变了对计算机化系统的检查方式,并出台了更新的法规(6,7)和指南(3, 8–11),我们接下来将对此进行讨论。(关注点2:纸质打印件可以篡改而不被记录,电子记录的篡改有审计追踪强制记录篡改)
What the Regulators Want
监管机构想要什么
In addition to providing a definition of hybrid system, the WHO good records management practices guidance also notes, in Appendix 1, under special risk factors in the Attributable section (3):
除了对混合系统进行定义外,世界卫生组织(WHO)良好记录管理规范指南还在附录1的“可追溯性”部分中关于特殊风险因素的内容下指出(3):
The use of hybrid systems is discouraged, but where legacy systems are awaiting replacement, mitigating controls should be in place.
不鼓励使用混合系统,但在现有旧系统尚待更换的情况下,应实施相应的缓解控制措施。
In such cases, original records generated during the course of [good practice] GXP activities must be complete and must be maintained throughout the records retention period in a manner that allows the full reconstruction of the GXP activities.
在这种情况下,于药品生产质量管理规范(GXP)活动过程中产生的原始记录必须完整,并且必须以允许完整重现该GXP活动的方式,在整个记录保存期间予以妥善保存。
A hybrid approach might exceptionally be used to sign electronic records when the system lacks features for electronic signatures, provided adequate security can be maintained. The hybrid approach is likely to be more burdensome than a fully-electronic approach; therefore, utilizing electronic signatures, whenever available, is recommended.
在系统不具备电子签名功能的情况下,可例外地采用混合方式对电子记录进行签署,但前提是必须能够维持足够的安全性。与完全电子化的方式相比,混合方式可能带来更大的管理负担;因此,建议在条件具备时优先使用电子签名。(关注点3:出现混合系统的原因之一是系统不具备电子签名功能)
Replacement of hybrid systems should be a priority.
应优先替换混合系统。
Have you got the message? Hybrid systems are not liked, and using them to ensure data integrity will cost you time and effort. The WHO guidance goes about as far as a regulatory guidance can go by stating that hybrid systems are discouraged, and that they should be replaced as a matter of priority. The Pharmaceutical Inspection Co-operation Scheme (PIC/S) PI-041 Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments also notes in section 9.3 (10):
你明白了吗?混合系统并不受欢迎,若要依靠它们来确保数据完整性,将会耗费你大量时间和精力。世界卫生组织(WHO)的指南已经近乎达到了监管指南所能表述的极限,明确指出不鼓励使用混合系统,并强调应优先予以替换。此外,《药品检查合作计划》(PIC/S)在《受监管的GMP/GDP环境中数据管理和完整性的良好规范》(PI-041)第9.3节中也指出(10):
Increased data review is likely to be required for hybrid systems.
混合系统可能需要进行更加强化的数据审核。
This is true as a second person reviewing hybrid records must review both paper and electronic records plus the linkages between the two types of records as shown in Figure 1, making the review more labor intensive and slow. As Newton and McDowall noted, the second person review may take longer than the actual chromatographic run (12).
确实如此,因为对混合记录进行第二人审核时,必须同时审查纸质记录和电子记录,以及这两类记录之间的关联性(如图1所示),这使得审核过程更加耗时费力且速度缓慢。正如牛顿(Newton)和麦克道尔(McDowall)所指出的,第二人审核所需的时间甚至可能超过实际色谱运行的时间(12)。(关注点4:混合系统审核的内容包括电子和纸质,包含二者之间的关联)
The World Consists of Hybrid Systems
世界充斥着混合系统
OK, saying that the world consists of hybrid systems is not quite accurate, but, in many laboratories, standalone hybrid computerized systems are used. Even networked systems with the technical controls to be used electronically can be deployed in hybrid mode, because this approach is seen as a tried and trusted option. This viewpoint is wrong, and dangerously so.
好吧,说整个世界都由混合系统构成虽有些夸张,但确实在许多实验室中,独立运行的混合型计算机化系统仍在被广泛使用。甚至一些具备电子化操作所需技术控制功能的网络化系统,也常常以混合模式部署,因为人们认为这种方式是经过验证且值得信赖的。然而,这种观点是错误的,而且非常危险。
There are now more stringent controls placed on hybrid systems, especially if it is a standalone workstation. Regulatory guidance notes that hybrid systems will probably result in more review work compared with an electronic process, and at the same time regulatory exposure will increase over time as interpretation becomes tighter. By failing to replace hybrid systems, management must accept accountability for its inaction when an inspector calls and finds a data integrity problem. To understand the situation from the perspective of the regulations, we need to go back in time to the last century.
如今,对混合系统(尤其是独立工作站)的管控已变得更加严格。监管指南指出,与完全电子化的流程相比,混合系统可能会导致更多的审核工作量,同时,随着监管解释日益严格,企业的合规风险也将随着时间推移而增加。如果未能及时替换混合系统,当检查员发现数据完整性问题时,管理层必须为其不作为承担相应的责任。要从法规的角度理解当前的形势,我们需要回溯到上个世纪。
Understanding the "c" in cGMP
Have you ever wondered why the FDA Good Manufacturing Practice (GMP) regulations have the word current in the title? To find out why we need to go back in time to September 29, 1978, and the publication of FDA's Current Good Manufacturing Practice (cGMP) regulations in the Federal Register (13). You may ask why we should bother with something that was published in the mists of time, when all we need to do is an Internet search as find the GMP regulations on the web. Not quite, dear reader. If you bother to read the regulations you will find that there is no definition or explanation of the word current in the regulation (for the purists, there is also no mention of current in the definitions of cGMP in 21 CFR 210).
理解 cGMP 中的 "c"
你是否曾好奇过,为什么美国食品药品监督管理局(FDA)的《药品生产质量管理规范》(GMP)法规标题中会加上“现行”(current)一词?要解答这个问题,我们需要回到1978年9月29日,那一天,FDA 在《联邦公报》(Federal Register)上发布了《现行药品生产质量管理规范》(Current Good Manufacturing Practice, cGMP)法规(13)。
你可能会问:我们为什么要关心几十年前发布的文件?毕竟现在只需上网搜索,就能轻松找到 GMP 法规。但情况并非如此简单,亲爱的读者。如果你真的去查阅这些法规原文,就会发现法规中并未对“current”一词进行定义或解释(对于考究细节的读者而言,在《美国联邦法规》第21篇第210部分(21 CFR 210)关于cGMP的定义中,也没有提及“current”这个词)。
那么,“c”到底意味着什么?尽管法规文本中没有明确定义,但“current”实际上体现了 FDA 的一项核心理念:制药企业必须采用当前科学和技术水平下合理的、最新的实践和标准来确保产品质量和患者安全。这意味着 GMP 不是一套静态、一成不变的规则,而是随着科学进步、技术发展和行业认知的提升而不断演进的动态要求。企业不能仅仅满足于“过去一直这么做”,而必须持续评估并采用更先进、更可靠的方法和系统——例如,从存在风险的混合系统转向更安全、可追溯性更强的全电子化系统。
因此,“c”不仅是一个字母,更是一种责任,它要求企业与时俱进,主动管理风险,确保数据完整性和产品质量。这也正是为何监管机构如今强烈建议淘汰混合系统、拥抱现代化电子记录和签名技术的根本原因。
The way that current was intended to be used will be found in preamble comment 17 of the regulations published in 1978 (13). It is built into the GMP regulation from the beginning, and not slipped in as an afterthought. In the scope section of the regulation (21 CFR 211.1), it states that:
“current”一词的 intended 用法,可以在1978年发布的法规前言部分的第17条说明中找到(13)。这个词从一开始就被融入GMP法规之中,而非事后临时添加。在法规的适用范围部分(21 CFR 211.1)中明确指出:
The regulations in this part contain the minimum current good manufacturing practice for preparation of drug products for administration to humans or animals.
(a) 本部分的法规包含了为供人类或动物使用的药品生产所规定的最低限度的现行药品生产质量管理规范。
The 21 CFR 211 regulations are stated to be the minimum expected. The problem with the pharmaceutical industry is that these regulations are typically interpreted as "These directives are all we will do." This divergence from the intent of the regulation is the start of some data integrity problems. We can delve further into an understanding of current in comment 17 of the 1978 preamble to the GMP regulations (13); here is a discussion on the use and meaning of the word current is as follows:
21 CFR 211 法规被明确表述为所期望的最低标准。制药行业存在的一个问题在于,这些法规通常被解读为“这些指令就是我们仅需完成的全部工作”。这种对法规本意的偏离,正是某些数据完整性问题的根源之一。我们可以进一步探究1978年GMP法规前言部分第17条评论(13)中对“current”一词的使用和含义的阐述;相关内容讨论如下:
One comment recommended that the word “current” be deleted since it is obvious that the latest regulations to be published are current, and therefore the use of the word “current” is superfluous....
有评论建议删除“current”(现行)一词,理由是显而易见的:最新发布的法规本身就是现行的,因此使用“current”一词是多余的……
Several of these comments reflect, the Commissioner believes, a misunderstanding regarding the use of the word “current”....
委员会认为,这些建议中的若干条反映了对“current”(现行)一词使用方式的误解……
The Congress intended that the phrase itself have a unique meaning and that the good manufacturing practice regulations represent sound current methods, facilities and controls for the production of drugs to assure safety…
国会的本意是,该短语本身应具有特定的含义,即药品生产质量管理规范应代表能够确保药品安全的、科学合理的现行方法、设施和控制措施……
Although the practices must be “current” in the industry, they need not be widely prevalent. Congress did not require that a majority or any percentage of manufacturers already be following the proposed mandated practices, as long as it was a current good manufacturing practice in the industry, i.e. that it had been shown to be both feasible and valuable in assuring drug quality (13).
尽管这些规范在行业内必须是“现行”的,但并不要求它们已被广泛采用。国会并不要求大多数或一定比例的生产商已经实施了这些被强制推行的规范,只要该规范在行业内属于现行的优良制造实践即可,也就是说,只要已证明该实践在确保药品质量方面既可行又有价值(13)。
The intent of the US GMP regulations has been that as science, analytical instrumentation, and technologies advance, then so too must the pharmaceutical industry. Even if an advance is not widely used in the industry, if it can ensure an improvement in drug quality, then it comes under the purview of the "c" in cGMP.
美国GMP法规的本意是:随着科学、分析仪器和技术的进步,制药行业也必须随之发展。即使某项技术进步在行业内尚未被广泛采用,只要它能够确保药品质量的提升,就属于cGMP中“c”(现行)的范畴,企业就应考虑采纳。这体现了cGMP动态、前瞻性的本质——合规不仅仅是满足最低书面要求,更是持续追求更高标准以保障患者安全和产品质量的责任。
However, the problem is that reality in regulated laboratories does not match regulatory intent. Once a pharmaceutical organization has developed an interpretation of the regulation, it is reluctant to change for several reasons, such as the cost of new technology, validation costs, the cost of updating a registration dossier, and perceived or actual inflexibility of the inspectorate to accept such advances. The companies often prefer to remain with older processes that do not reflect the most effective and efficient ways of working, if they have not had any problems in the past.
然而,问题在于受监管实验室的现实情况与监管的初衷并不相符。一旦制药企业对法规形成了某种解释或执行模式,出于多种原因,它们往往不愿做出改变,例如:引进新技术的成本、验证所需的成本、更新注册申报资料的费用,以及认为或实际遇到监管检查部门对新技术接受度低、缺乏灵活性等问题。因此,企业通常倾向于继续沿用过去没有出过问题的旧有流程,即使这些流程已不能代表最有效、最高效的工作方式。这种惯性阻碍了行业采用更先进的实践,违背了cGMP中“c”(现行)所倡导的持续改进精神,最终可能导致数据管理效率低下、合规风险上升,甚至在检查中暴露数据完整性缺陷。
Personal computers have been used for controlling chromatographs since the 1980s, and they have typically been designed to be used in the same way, as a hybrid system. Publication of 21 CFR 11 regulations (14) in 1997 allowed the use of electronic signatures, but widespread adoption has not occurred. Large pharmaceutical companies have implemented site or global CDS with electronic signatures, but smaller companies typically have not gone in the same direction, preferring to have standalone systems, often from several different suppliers, which can be argued as crass stupidity.
自20世纪80年代以来,个人计算机已被用于控制色谱仪,而这些系统在设计上通常被用作混合系统。1997年发布的《21 CFR 第11部分》法规(14)允许使用电子签名,但该技术的广泛应用并未实现。大型制药公司已在其站点或全球范围内实施了支持电子签名的色谱数据系统(CDS),但小型公司通常未采取相同方向,反而倾向于使用独立的系统,且这些系统往往来自多个不同的供应商。这种做法可以说是极其愚蠢的。
Hybrid System Configuration and Data Integrity Issues
混合系统配置与数据完整性问题
A typical hybrid system is shown in Figure 2 and consists of three components:
典型的混合系统如图2所示,包含三个组成部分:
Analytical instrument, such as a chromatograph
分析仪器,如色谱仪
Controlling workstation, where the CDS software is loaded and where data are stored
控制工作站,即安装了色谱数据系统(CDS)软件并用于存储数据的计算机。
Printer
打印机
Figure 2 shows the potential data integrity problems that fall into three categories: business efficiency and continuity, IT data integrity, and laboratory data integrity. One of the major problems with hybrid systems is protecting the data and associated metadata with effective backup and recovery processes. If backup is left to the laboratory, it will inevitably fail, because backup is not the main function of a chromatography laboratory.
图2显示了潜在的数据完整性问题,可分为三类:业务效率与连续性、IT数据完整性以及实验室数据完整性。混合系统的一个主要问题是,无法通过有效的备份和恢复流程来保护数据及相关元数据。如果将备份工作交由实验室负责,则必然失败,因为备份并非色谱实验室的主要职能。
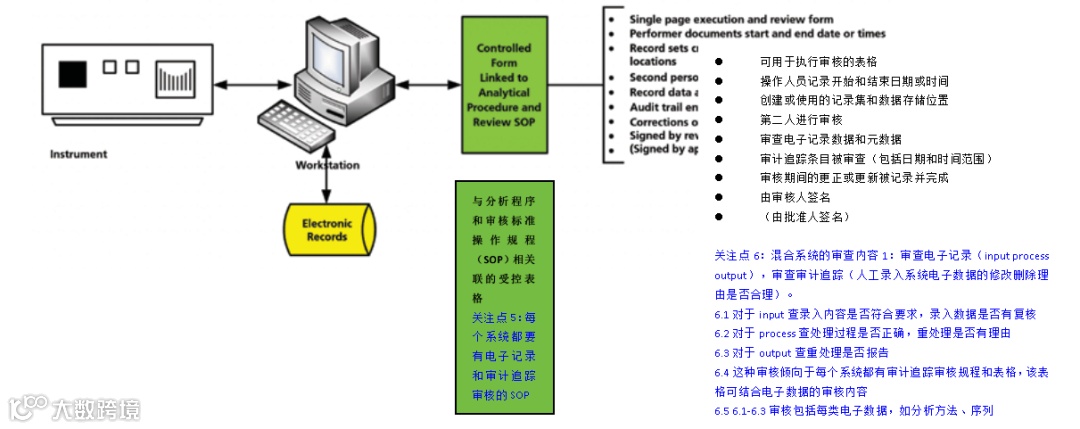
Figure 2: Typical configuration of a hybrid chromatography data system and potential data integrity problems (4).
图2:混合色谱数据系统的典型配置与潜在的数据完整性问题(4)。
Are You Lazy?
你懒吗?
Most hybrid systems print paper as if it is going out of fashion. Depending on the sadists in the Quality Assurance (QA) department, often each page must be initialled by the tester, and again by the reviewer, which is an error prone, tedious, and non-value-added task. The printouts must also be checked against the electronic records that were created or used in the analysis.
大多数混合系统打印纸质文件,仿佛纸张即将过时。根据质量保证(QA)部门中那些“施虐者”的要求,通常每一页都必须由检测人员签名,再由审核人员重复签名,这是一种容易出错、枯燥且不增加价值的任务。此外,还必须将打印件与分析过程中创建或使用的电子记录进行核对。(关注点7:混合系统审核内容2:电子记录和纸质记录一致,确保纸质记录未被篡改。结合关注点6,应重点审核电子记录,纸质记录审核仅关注纸质和电子一致性,毕竟纸质打印出来的在电子记录中一定会有,之所以强调纸质和电子记录的关联,也是审核时需要去通过关联来审核一致。)
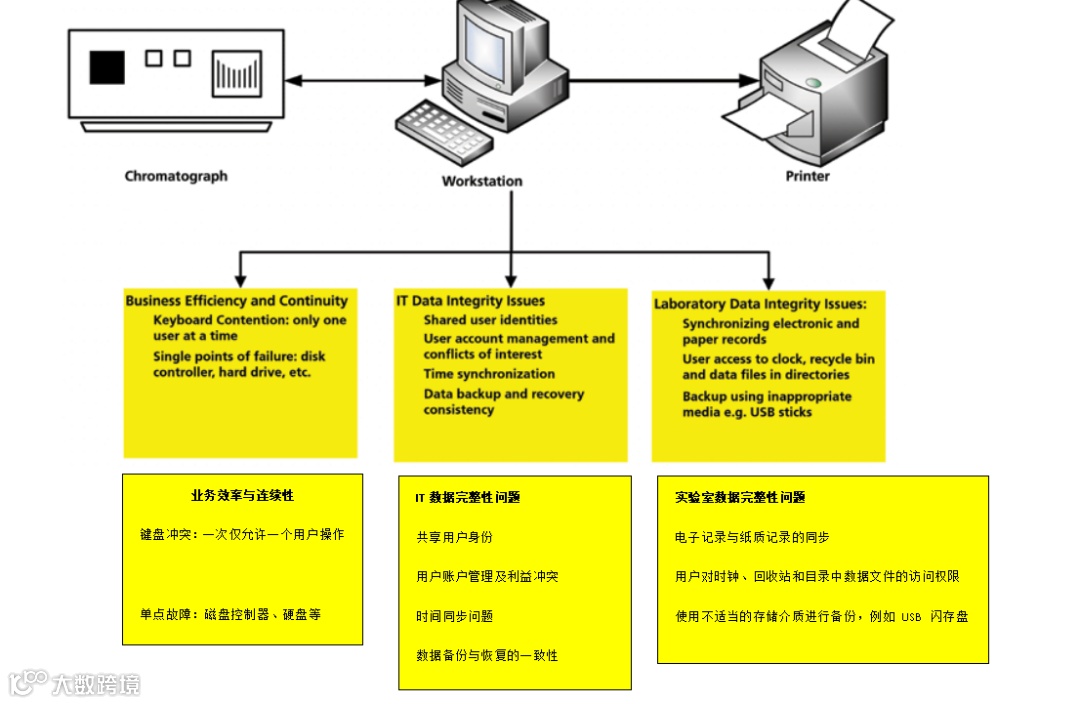
Figure 3: A schematic of the generation and review of hybrid records (4).
A simple way to reduce the amount of paper printed from a hybrid system is outlined in Appendix 1 of the WHO data integrity guidance (3). Shown in Figure 3 is a controlled and uniquely numbered review form (10,15) for the creation and review of the records created during an analysis using a hybrid system. The form is linked to the analytical procedure and standard operating procedure (SOP) for review of laboratory records. The steps are as described below.
世界卫生组织(WHO)数据完整性指南附录1(3)中概述了一种减少混合系统打印纸张数量的简单方法。图3显示了一种受控且具有唯一编号的审查表单(10,15),用于在混合系统下对分析过程中生成的记录进行创建和审查。该表单与分析方法及实验室记录审查的标准操作规程(SOP)相关联。具体步骤如下所述。
At the start of the analysis, a uniquely numbered version of the form is issued to the tester, who documents the start and end date and time of the analysis (to help the second-person reviewer search audit trail entries), as well as documents the records created, modified, and the location where they are stored.
分析开始时,向检测人员发放一份具有唯一编号的表单,由其记录分析的开始和结束日期及时间(以帮助第二人审核员查找审计追踪记录),并记录所创建、修改的文件以及这些文件的存储位置。
Chromatograms and data are reviewed by the analyst on screen. The only printout from the CDS is the test summary of the reportable results and analysis information.
分析人员在屏幕上审阅色谱图和数据。从色谱数据系统(CDS)中唯一打印的文件是报告结果和分析信息的测试摘要。(关注点8:减少打印内容,以减少交叉审核的内容)
The results printout and review form is signed by the tester.
检测人员对结果打印件和审查表单进行签名。
When records are ready for review, a second analyst reviews files electronically on screen with no printouts; this makes the task simpler and quicker.
当记录准备就绪进行审核时,第二位分析人员在屏幕上以电子方式审阅文件,无需打印件;这使得审核工作更简单、更快速。
The reviewer checks the data files and contextual metadata files generated by the performer of the test, and documents these on the review form. The applicable audit trail entries are also reviewed between the analysis start and end times and dates, and documented.
审核人员检查由检测人员生成的数据文件和相关的元数据文件,并将这些信息记录在审查表单上。同时,审核人员还需审查分析开始至结束时间范围内的相关审计追踪记录,并进行记录。
Checks for falsification of data are included in the form, as this will be reviewed during data integrity audits and, if applicable, data integrity investigations.
审查表单中包含了对数据伪造的检查项(关注点9:应当是图3中说的审核电子记录与纸质记录同步性),因为该表单将在数据完整性审计期间被审查,如适用,也将在数据完整性调查中被核查。
If changes are required to be made, these are documented and sent back to the tester to update. When the review is complete, the reviewer then signs the form. If required by laboratory procedures, there may be space on the form for an approval or QA signature.
This approach reduces the volume of paper printed, but also allows a faster review, as the cross check between the electronic and paper is far smaller than now.
如果需要进行修改,相关意见将被记录下来并退回给测试人员进行更新。当审核完成后,审核人再在表单上签字。如果实验室规程有要求,表单上也可留有供批准人或质量保证(QA)人员签名的位置。
这种方法减少了打印纸张的使用量,同时也加快了审核流程,因为电子记录与纸质记录之间的交叉核对工作量远比目前的做法要小得多。
(关注点10:这里介绍了一个很好的电子数据审核+审计追踪审核的案例)
Eliminate Hybrid Systems
淘汰混合系统
Working with hybrid systems–computerized systems that generate electronic records with signed paper printouts–is the worst possible world to be in. The laboratory must manage two incompatible media formats: paper and electronic records. The best advice is to eliminate these systems by using electronic systems to ensure both regulatory compliance and business efficiencies, as we discussed in the second part of this series (2).
使用混合系统——即生成电子记录但以签署的纸质打印件(关注点11:系统没有审计追踪且数据可修改也是打印数据的一种场景,这种场景下简化打印内容还可以做到防篡改吗?)作为正式记录的计算机化系统——是最糟糕的选择。实验室必须同时管理两种互不兼容的介质形式:纸质记录和电子记录。最好的建议是淘汰这类系统,转而采用全电子化系统,以确保既符合法规要求又能提升业务效率,这一点我们在本系列文章的第二部分中已作讨论(2)。
Why Are You Still Buying Hybrid Systems?
I could have entitled this subheading "Why are suppliers still selling hybrid systems?" However, given that suppliers respond to market forces, it is the responsibility of customers to put pressure on suppliers to design adequate systems for today's data integrity world. Laboratories that have assessed and remediated current systems are perpetuating the problem when new systems are purchased, because they are typically:
我本可以将这个小标题定为“为什么供应商仍在销售混合系统?”。然而,鉴于供应商是顺应市场力量而行的,客户有责任向供应商施加压力,要求他们为当今数据完整性需求设计出完善的系统。那些已经对现有系统进行过评估和整改的实验室,在购买新系统时却仍在延续这一问题,原因通常是:
standalone独立运行的系统(关注点12:应当说的是仪器采集数据而不能存储数据的情况)
hybrid混合系统
involve data being stored in directories created in the operating system rather than in a database数据存储于操作系统中创建的目录(如文件夹)内,而非存储在数据库中(关注点13:数据存储在文件夹内,删改没有经过软件的审计追踪记录,造成不可控;数据存储在数据库从windows层面当然也可删,但删除整个数据库很容易被发现)
For further discussion regarding CDSs, please refer to the four-part series written for LCGC North America by Chris Burgess and myself (16–19).
有关色谱数据系统(CDS)的进一步讨论,请参阅由Chris Burgess和我本人为《LCGC北美版》撰写的四部分系列文章(16–19)。
The Year-End Slush Fund Spend?
年终“应急资金”挥霍?
You know the situation: A month before the end of the financial year, your boss puts his or her head round the door of the laboratory and says nonchalantly, "We have money to spend before the year end. Any ideas?" This question initiates a mad panic, because three quotes have to be generated, the capital request raised and walked around for signature, the purchase order raised, and an empty box delivered to stores. What a quality way to purchase instrumentation and software. It is extremely unlikely that the overall system has been adequately assessed for compliant functions and technical controls for data integrity. The staff only focus on the bright shiny instrument. However, this reaction perpetuates the data integrity problem. But this problem always occurs in other organizations, doesn't it?
你一定很熟悉这种情况:在财年结束前一个月,你的老板把头探进实验室,漫不经心地说:“我们在年底前还有钱没花完,有什么想法吗?”这个问题一出,立刻引发一阵疯狂的慌乱:必须准备三份报价,提交资本支出申请并四处找人签字,然后开出采购订单,最后还得让一个空箱子被送到仓库。这就是所谓“高质量”地采购仪器和软件的方式。在这种情况下,几乎不可能对整个系统进行充分评估,以确认其是否具备符合要求的合规功能和保障数据完整性的技术控制措施。员工们只关注那台光鲜亮丽的新仪器。然而,这种做法却 perpetuates(延续了)数据完整性问题。但这个问题,总是发生在别的组织里,对吧?
Summary
We have defined what a hybrid system is, and why it is not recommended by regulatory authorities. The biggest problem with hybrid systems is the synchronization of two incompatible media. However, the hybrid problem is perpetuated by dumb suppliers who do not design software for electronic working and data acquisition directly to a network storage area. The problem is compounded by stupid laboratories that keep purchasing applications with inadequately designed compliance functions to give data integrity assessors a job for life.
我们已经定义了什么是混合系统,以及为何监管机构不推荐使用混合系统。混合系统最大的问题在于两种不兼容介质之间的同步。然而,这一问题却被一些愚蠢的供应商所延续,他们并未设计出可直接将电子数据采集到网络存储区域的软件。这个问题还因一些愚昧的实验室不断购买合规功能设计不完善的软件应用而加剧,这反倒为数据完整性审查人员提供了终身的工作机会。
暑热褪去,秋凉渐至,各类花仙子在争奇斗艳,热情洋溢的向日葵,明媚灿烂的格桑花,蜂飞蝶舞,小火车像个调皮的孩童转来转去,无人机高高低低转着圈欣赏着这副油墨山水画。大隐了一个漫长的夏天,来个小隐吧。


